chevron down SecretStupid 09/27/2020 Chemistry College answer answered Aluminum has a density of 2.70 g/cm^3. How Many moles of aluminum are in a 13.2 cm^3 block of the metal substance? Fill in the template rotate Advertisement Answer 4 people found it helpful profile Brainly User 2 Answer: 1.32 MOLES 35.64G / 27G/MOLE
Solved The accepted density of aluminum is 2.70 g/mL. Two | Chegg.com
Expertverified 100% (2 ratings) Step 1 Here, given data ρ 0 = 2.7 g / c m 3 T 0 = 20 0 C T = 500 0 C Δ T = ( 500 − 20) = 480 0 C View the full answer Answer Unlock Previous question Next question Transcribed image text: Aluminum has a density of 2.70 g/cm^3 at room temperature (20 degree C). Determine its density at 500 degree C.
Source Image: chegg.com
Download Image
Aluminum has a density of 2.70 g/cm3. What would be the mass of a sample whose volume is 10.0 cm3? Chemistry Measurement Density 1 Answer Stefan V. Apr 26, 2017 27.0 g Explanation: The density of a substance tells you the mass of exactly one unit of volume of said substance. In your case, aluminium is said to have a density of

Source Image: scribd.com
Download Image
High Pure Aluminum Grains Pellets 99.999% 3mm High Density Metal Aluminum Al Aluminum Pellets Price – China Aluminum Granule, High-Quality Aluminum Particles | Made-in-China.com Get the right answer, fast. Ask a question for free Get a free answer to a quick problem. Most questions answered within 4 hours. OR Find an Online Tutor Now Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Aluminum has a density of 2.70 g/cm3.

Source Image: bartleby.com
Download Image
Aluminium Has A Density Of 2.70 G/Cm3
Get the right answer, fast. Ask a question for free Get a free answer to a quick problem. Most questions answered within 4 hours. OR Find an Online Tutor Now Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Aluminum has a density of 2.70 g/cm3. 1 Answer Chuck W. Jun 7, 2016 The mass of an object is equal to the product of its density and volume. Check to ensure that the units cancel properly. Explanation: 2.70 g cm3 ×1.50cm3 = 4.05g Answer link The mass of an object is equal to the product of its density and volume.
Answered: Aluminium has a density of 2.70 g/cm.… | bartleby
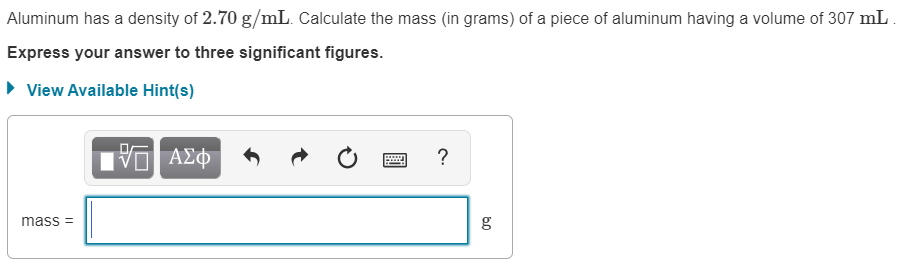
Step 1 We know that D e n s i t y = M a s s V o l u m e M a s s = D e n s i t y × V o l u m e View the full answer Answer Unlock Previous question Next question Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved aluminum has a density of 2.70 g/cm3. What is the | Chegg.com Solved Aluminum has a density of 2.70 g/mL. Calculate the | Chegg.com
Source Image: chegg.com
Download Image
b) The density of aluminum is 2.70 g/cm3. The thickness of a rectangular sheet of aluminum foil varies – brainly.com Step 1 We know that D e n s i t y = M a s s V o l u m e M a s s = D e n s i t y × V o l u m e View the full answer Answer Unlock Previous question Next question Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved aluminum has a density of 2.70 g/cm3. What is the | Chegg.com

Source Image: brainly.com
Download Image
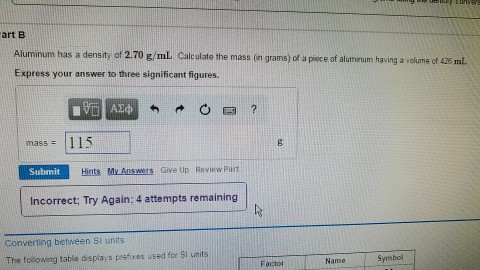
Solved The accepted density of aluminum is 2.70 g/mL. Two | Chegg.com chevron down SecretStupid 09/27/2020 Chemistry College answer answered Aluminum has a density of 2.70 g/cm^3. How Many moles of aluminum are in a 13.2 cm^3 block of the metal substance? Fill in the template rotate Advertisement Answer 4 people found it helpful profile Brainly User 2 Answer: 1.32 MOLES 35.64G / 27G/MOLE
Source Image: chegg.com
Download Image
High Pure Aluminum Grains Pellets 99.999% 3mm High Density Metal Aluminum Al Aluminum Pellets Price – China Aluminum Granule, High-Quality Aluminum Particles | Made-in-China.com Aluminum has a density of 2.70 g/cm3. What would be the mass of a sample whose volume is 10.0 cm3? Chemistry Measurement Density 1 Answer Stefan V. Apr 26, 2017 27.0 g Explanation: The density of a substance tells you the mass of exactly one unit of volume of said substance. In your case, aluminium is said to have a density of

Source Image: 48bc1abfae09393c.en.made-in-china.com
Download Image
Presentation – Aluminium | PDF | Aluminium | Metals Solid aluminum forms a face-centered cubic unit cell. Aluminum has a density of $\pu2.70 g/cm^3$. Determine the edge length of the $\ceAl(s)$ unit cell in $\pucm$. Usually if I enter the wrong answer the it will show all the calculations to determine the correct answer. However this one does not show the calculations. Here is the work I

Source Image: scribd.com
Download Image
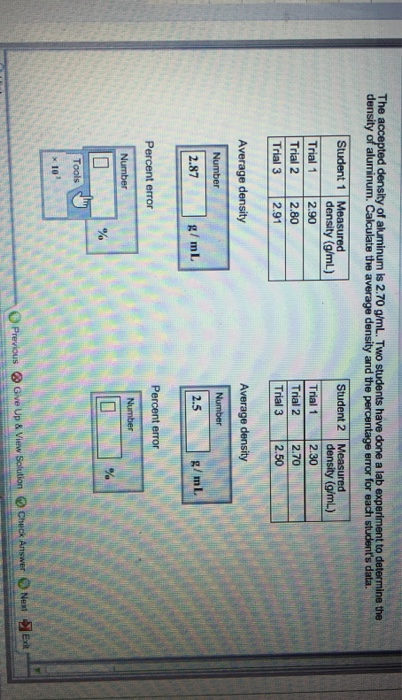
SOLVED: HELP ASAP PLEASE! THIS IS FOR STUDY ISLAND Metal Density (g/cm3) aluminum 2.70 zinc 7.14 iron 7.87 copper 8.96 silver 10.49 lead 11.34 mercury 13.55 gold 19.32 6 What is the Get the right answer, fast. Ask a question for free Get a free answer to a quick problem. Most questions answered within 4 hours. OR Find an Online Tutor Now Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Aluminum has a density of 2.70 g/cm3.
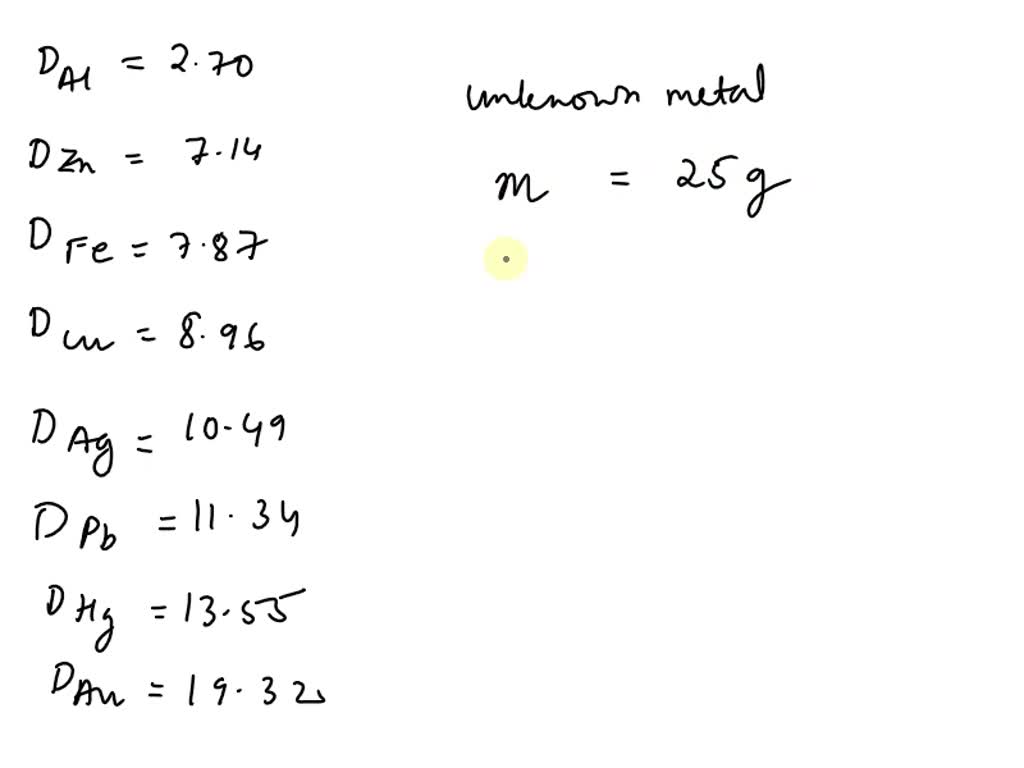
Source Image: numerade.com
Download Image
Aluminum has a density of 2.70 g/cm^3. How Many moles of aluminum are in a 13.2 cm^3 block of the metal – brainly.com 1 Answer Chuck W. Jun 7, 2016 The mass of an object is equal to the product of its density and volume. Check to ensure that the units cancel properly. Explanation: 2.70 g cm3 ×1.50cm3 = 4.05g Answer link The mass of an object is equal to the product of its density and volume.

Source Image: brainly.com
Download Image
b) The density of aluminum is 2.70 g/cm3. The thickness of a rectangular sheet of aluminum foil varies – brainly.com
Aluminum has a density of 2.70 g/cm^3. How Many moles of aluminum are in a 13.2 cm^3 block of the metal – brainly.com Expertverified 100% (2 ratings) Step 1 Here, given data ρ 0 = 2.7 g / c m 3 T 0 = 20 0 C T = 500 0 C Δ T = ( 500 − 20) = 480 0 C View the full answer Answer Unlock Previous question Next question Transcribed image text: Aluminum has a density of 2.70 g/cm^3 at room temperature (20 degree C). Determine its density at 500 degree C.
High Pure Aluminum Grains Pellets 99.999% 3mm High Density Metal Aluminum Al Aluminum Pellets Price – China Aluminum Granule, High-Quality Aluminum Particles | Made-in-China.com SOLVED: HELP ASAP PLEASE! THIS IS FOR STUDY ISLAND Metal Density (g/cm3) aluminum 2.70 zinc 7.14 iron 7.87 copper 8.96 silver 10.49 lead 11.34 mercury 13.55 gold 19.32 6 What is the Solid aluminum forms a face-centered cubic unit cell. Aluminum has a density of $\pu2.70 g/cm^3$. Determine the edge length of the $\ceAl(s)$ unit cell in $\pucm$. Usually if I enter the wrong answer the it will show all the calculations to determine the correct answer. However this one does not show the calculations. Here is the work I